- PI: Waltraud Schrottmaier
- Funded by: Anniversary Fund of the Austrian National Bank (OeNB18450)
- Funding period: 12/2020-05/2023
Recent severe outbreaks such as the West African Ebola virus epidemic with over 11.000 fatalities have put increased attention on viral haemorrhagic fevers (VHF). However, while VHFs such as Dengue and Ebola that mostly occur in Africa and Asia are well-known, awareness of Hantavirus-induced VHFs occurring in Europe and the Americas is low even though their mortality can reach 40%.
With about 2% mortality, the European Puumala Hantavirus (PUUV) is the mildest of the closely-related Hantaviruses, making PUUV the ideal candidate to study underlying mechanisms common to Hantavirus-induced VHFs. PUUV infection causes fever, temporary kidney failure and thrombocytopenia, leading to gastrointestinal bleedings but also to a higher risk for thrombosis.
Studying this double-edged effect on primary haemostasis and the resulting knowledge will help in the treatment of Hantavirus-infected VHF patients in order to limit potentially life-threatening haemorrhagic and thrombotic complications.
VHFs such as PUUV infection-caused Nephropathia epidemica (NE) are characterized by impaired haemostasis. As the cellular mediators of haemostasis, platelets are of pivotal importance for maintaining vascular integrity. Thus, low platelet counts and decreased platelet function – as occurring during acute NE – are associated with increased risk for bleeding.
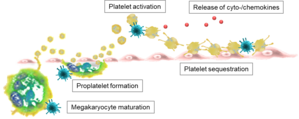
Although platelets play a central role in PUUV-induced haemorrhagic fever, it is currently unknown how PUUV infection causes thrombocytopenia and platelet dysfunction. Therefore, we aim to elucidate how infection with PUUV modulates platelet activation and platelet production.
We speculate that systemic PUUV infection activates platelets and induces endothelial adhesion and sequestration in capillary-rich organs, thereby reducing the number of circulating platelets. Further, PUUV infection may reduce the production of platelets by compromising maturation and/or differentiation of their progenitor cells in the bone marrow.
In order to investigate this, we are screening the blood of NE patients and performing in vitro experiments to evaluate the direct and indirect effects of PUUV on platelet production, function and activation.
We are convinced that the results of the study will be interesting for physicians and basic researchers alike as they will add significant knowledge to a more comprehensive view of the pathogenesis of Hantavirus-induced haemorrhagic fevers.
- PI: Waltraud Schrottmaier
- Co-PI with Prof. Bernd Jilma (MUW)
- Funded by: Medical Scientific Fund of the Mayor of the City of Vienna (BGM19029)
- Funding period: 06/2019-09/2020
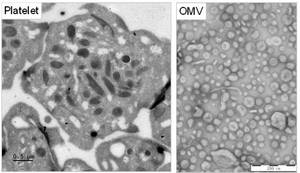
Cholera disease, caused by infection with the bacterium Vibrio cholerae, is a gastro-intestinal disease characterized by severe diarrhoea. Annually more than 2.8 million people worldwide are affected by cholera. Via shedding of outer membrane vesicles (OMV), V. cholerae is able to neutralize factors of the immune responses such as the complement system or antimicrobial proteins, enabling the pathogen to evade host immune defences. Furthermore, cholera infection may also lead to serious complications such as intestinal haemorrhages which drastically increase mortality.
As cellular mediators of haemostasis, platelets are of pivotal importance for maintaining vascular integrity by adhering to the damaged endothelium and sealing breaches of the vessel. Furthermore, platelets also respond to immune stimuli including bacterial infection. Thus, platelet dysfunction is generally associated with increased risk for bleeding and may be directly influenced by pathogens.
Although platelets play a central role in several haemorrhagic pathologies, there is currently little data available on how platelet-mediated primary haemostasis is affected by V. cholerae or their OMVs. Further the mechanisms underlying haemorrhagic complications during cholera infection remain unknown. Hence, we aim to elucidate how V. cholerae-derived OMVs modulate platelet activation.
Based on our preliminary data, we think that OMVs shed from V. cholera influence platelet function and regulate their capacity to form thrombi, which could lead to altered vascular integrity and may reflect haemostatic complications suffered by some V. cholera-infected patients.
Thus, we are investigating the effect of V. cholerae-derived OMVs on platelet activation in vitro. Further, we aim to identify the pathogenic factors involved in platelet-OMV interaction and their effect on platelet function.
Selected publications
- Platelet p110β mediates platelet-leukocyte interaction and curtails bacterial dissemination in pneumococcal pneumonia.
Schrottmaier WC, Kral-Pointner JB, Salzmann M, Mussbacher M, Schmuckenschlager A, Pirabe A, Brunnthaler L, Kuttke M, Maier B, Heber S, Datler H, Ekici Y, Niederreiter B, Heber U, Blomgren B, Gorki AD, Söderberg-Nauclér C, Payrastre B, Gratacap MP, Knapp S, Schabbauer G, Assinger A. Cell Rep. 2022 Nov 8;41(6):111614. - Platelets in Viral Infections - Brave Soldiers or Trojan Horses.
Schrottmaier WC, Schmuckenschlager A, Pirabe A, Assinger A. Front Immunol. 2022 Mar 28;13:856713. - Platelets and Antiplatelet Medication in COVID-19-Related Thrombotic Complications.
Schrottmaier WC, Pirabe A, Pereyra D, Heber S, Hackl H, Schmuckenschlager A, Brunnthaler L, Santol J, Kammerer K, Oosterlee J, Pawelka E, Treiber SM, Khan AO, Pugh M, Traugott MT, Schörgenhofer C, Seitz T, Karolyi M, Jilma B, Rayes J, Zoufaly A, Assinger A. Front Cardiovasc Med. 2022 Jan 24;8:802566. - Adverse Outcome in COVID-19 Is Associated With an Aggravating Hypo-Responsive Platelet Phenotype.
Schrottmaier WC, Pirabe A, Pereyra D, Heber S, Hackl H, Schmuckenschlager A, Brunnthaler L, Santol J, Kammerer K, Oosterlee J, Pawelka E, Treiber SM, Khan AO, Pugh M, Traugott MT, Schörgenhofer C, Seitz T, Karolyi M, Jilma B, Rayes J, Zoufaly A, Assinger A. Front Cardiovasc Med. 2021 Dec 10;8:795624. - Platelets mediate serological memory to neutralize viruses in vitro and in vivo.
Schrottmaier WC, Salzmann M, Badrnya S, Mussbacher M, Kral-Pointner JB, Morava S, Pirabe A, Brunnthaler L, Yaiw KC, Heber UM, Pereyra D, Andersen JT, Bergthaler A, Söderberg-Nauclér C, Karlsson MCI, Assinger A, Forsell MNE. Blood Adv. 2020 Aug 25;4(16):3971-3976.
