Selected publications
- Pereyra, D.; Heber, S.; Jilma, B.; Zoufaly, A.; Assinger, A., Routine haematological parameters in COVID-19 prognosis. Lancet Haematol 2020, 7 (10), e709.
- Schrottmaier, W. C.; Salzmann, M.; Badrnya, S.; Mussbacher, M.; Kral-Pointner, J. B.; Morava, S.; Pirabe, A.; Brunnthaler, L.; Yaiw, K. C.; Heber, U. M.; Pereyra, D.; Andersen, J. T.; Bergthaler, A.; Soderberg-Naucler, C.; Karlsson, M. C. I.; Assinger, A.; Forsell, M. N. E., Platelets mediate serological memory to neutralize viruses in vitro and in vivo. Blood Adv 2020, 4 (16), 3971-3976.
- Schrottmaier, W. C.; Mussbacher, M.; Salzmann, M.; Assinger, A., Platelet-leukocyte interplay during vascular disease. Atherosclerosis 2020, 307, 109-120.
Current research projects
- PI: Alice Assinger
- Funded by: Austrian Science Fund (FWF- P 32064)
- Funding period: 9/2020-9/2023
The liver is the only organ which is able to restore its own mass after large parts have been removed. This makes liver resection a widely used and effective treatment for many cancers.
Despite improvements in surgical techniques and patient care, postoperative morbidity and mortality after liver resection remain a major concern, leading to liver failure and death in 11-13% of high-risk patients. There is currently no reliable marker to predict liver complications, nor are there any strategies to improve disease progression.
For several years we have been investigating novel methods for detection as well as improvement of therapeutic interventions to prevent liver dysfunction.
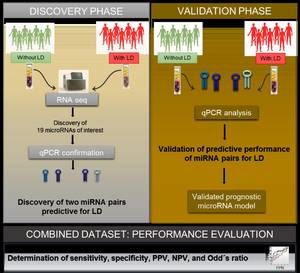
Recently, we made a significant breakthrough to predict postoperative liver regeneration complications: we could show that microRNAs, which circulate in blood, can predict complications in liver regeneration with a better accuracy than any previously available parameter for liver function. In this project, we plan to evaluate the therapeutic potential of microRNAs in liver regeneration, in order to develop novel treatment strategies for patients. To explore this, we apply modern in vitro techniques as well as animal models to investigate the therapeutic role of microRNAs in liver regeneration processes.
The fact that there is currently no treatment option available to support liver regeneration is a major clinical problem. Thus, this project is highly relevant to improve the quality of life and survival of these patients.
- PI: Alice Assinger
- Funded by: ACOVACT study of the Medical University Vienna is financially supported by the Austrian Federal Ministry of Education, Science and Research
- Funding period: 12/2020-6/2022
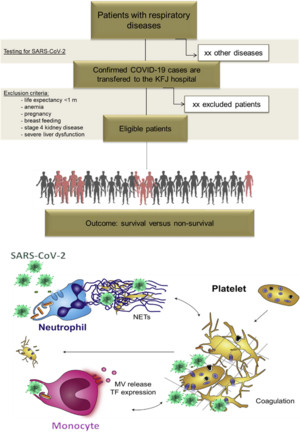
Coronavirus disease 2019 (COVID-19) is a novel infectious disease, which can cause acute respiratory distress syndrome (ARDS), liver failure and multi-organ failure in 2-15 % of patients. Although the lung is the primary affected organ, several studies revealed that COVID-19 patients are also at severe risk for thrombotic complications. The features of COVID-19-associated coagulopathy are unique and incompletely understood. In this project we study the impact of coagulation- and platelet-associated parameters in a prospectively collected patient cohort with COVID-19.
- PI: Alice Assinger
- Co-PI with Christa Firbas (MUW)
- Funded by: Medical Scientific Fund of the Mayor of the City of Vienna (COVID024)
- Funding period: 06/2020-12/2021
Coronavirus disease 2019 (COVID-19) is a novel infectious disease caused by SARS-CoV-2 virus, which can cause severe acute respiratory syndrome and lead to lung failure and multi-organ failure in 2-15 % of patients.
In order to optimize patient care and resource allocation during this pandemic, biomarkers are urgently needed for stratifying patients’ risk and for actively monitoring illness severity.
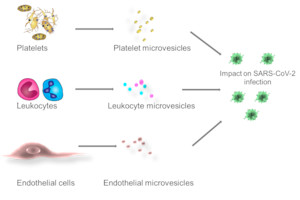
Emerging evidence suggests that microvesicles, small molecules shed from different (blood) cells, play a pivotal role in various diseases. Microvesicles are e.g. major carriers of plasma microRNAs that can change cellular functions of target cells. Changes in microvesicle quantity and composition have been observed in diseased patients and have been postulated as surrogate marker for various diseases.
In this project we aim to investigate whether the circulating microvesicle signatures change during COVID-19 and evaluate if microvesicle alterations are associated with disease severity. As inflammatory cytokines, e.g. tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), and IL-8, are elevated in other respiratory syndromes we will also monitor these cytokines. Moreover we will analyse if microvesicle signatures and inflammatory cytokines show gender specific alterations and co-incide with pre-existing conditions.
We are currently collecting plasma from hospitalized patients with SARS-CoV-2 infection as well as plasma samples from age and gender matched controls. Usage of CTAD as anticoagulant, immediate plasma preparation and our two-step centrifugation protocol ensure high quality samples. We have well equipped laboratories and all techniques established that allow for comprehensive microvesicle analysis. Materials that allow us to perform this analysis are subject of this proposal.
The results of this study are supposed to add significant knowledge to the systemic impact of SARS-CoV-2 and have the potential to provide a basis for a minimal invasive biomarker to allow for patients risk stratification and monitoring.
